12, Electricity and batteries
Batteries
The increasing reliance on renewable energy sources has created a demand for self-sustaining battery power. Rechargeable batteries operating through chemical reactions are commonly used for renewable energy storage.
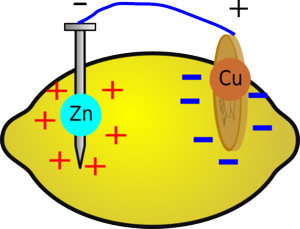
Below we're going to look at two different batteries. The first, the lemon battery is not rechargeable, the second, uses copper and aluminium and is rechargeable.
Lemon battery
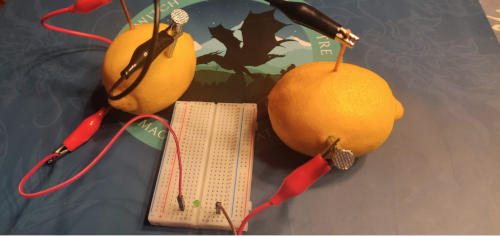 Ingredients:
Ingredients:
- 4 lemons
- 4 galvanized nails - 2 inches or 4 cm long
- 4 copper nails / wire - 2 inches or 4 cm long
- Some connectors
- low voltage LED light
Instructions:
Soften the lemon by gently pressing down on it rolling it around the table. This will get its juices flowing. However, don't damage the lemon by breaking the skin.
Insert one copper nail into the lemon. This is the positive side (called the anode).
Insert the galvanised nail into the other side. This is the negative side (called the cathode). Make sure the nails won't touch inside the lemon.
Do steps 1-3 with the other three lemons.
Connect the lemon batteries together as follows:
- Attach the first jumper wire to the galvanized nail of battery 1.
- Attach the second cable to the copper nail of battery 1, then attached the other side of this cable to the galvanized nail of battery 2.
- Attach one more cable to the copper nail of battery 2 to the galvanized nail of battery 3,
- Attache another cable connecting the copper nail of battery 3 to the galvanized nail of battery 4.
- Finally, attach the last cable to the copper nail of battery 4.
Your batteries are now strung together in series, and should produce around 4 volts direct current.
 .
. 
Take out your bread board.
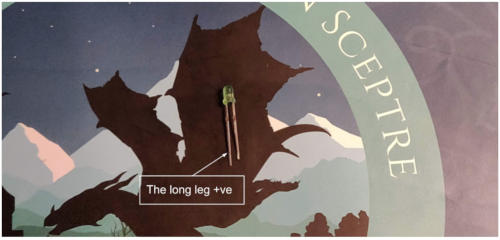
- Place the legs of the led on either side of the central canal. If you’re not sure how a breadboard works, you’ll find an explanation at the end of the book. Note which row has the long leg of the LED. This is the positive leg. LEDs work in a specific direction.
- Take 2 male connector cables and place them in any hole along the same row as the led legs (see the photo above)
Using two alligator clips, connect the positive cable of your batteries to the positive leg of the LED (the longest leg), and the negative cable to the negative leg (the short leg). Now you have a battery-powered light.

What if it doesn’t work?
Science is full of failures but it’s essential to know if you actually failed or there’s a problem. Here’s a list of all the things to check:
- The lemon segment walls may still be intact. For the battery to work, the walls separating the segments of the lemon need to be broken. That’s why we squeeze it to start.
- The circuit may be wired incorrectly. Check the circuit and make sure that for each battery, the negative silver nail is connected to the positive copper rod of the next battery. Otherwise the electricity won’t flow. Electricity always flows from positive to negative.
- Make sure the positive battery terminal is connected to the long (positive) leg of the LED.
- Swap the LED to make sure it’s not broken.
- Check the voltage with a volt meter. You need at least 2 volts to get light, 3-4 volts is better.
If all that fails, contact us, we’d love to hear from you and help.
 We get about 1 volt from a single lemon - not bad! A aaa battery gives around 1.5 volts, but is way more convenient.
We get about 1 volt from a single lemon - not bad! A aaa battery gives around 1.5 volts, but is way more convenient.
With two lemons, we get low light.
What is happening?
(Curtesy, thenakedscientists.com: https://www.thenakedscientists.com/get-naked/experiments/lemon-powered-ipod-fruit-batteries
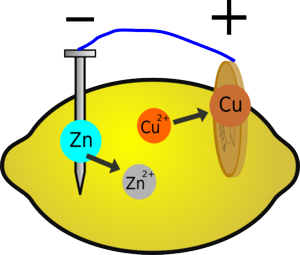
When you insert the two different metals into the fruit, a chemical reaction will occur. Metallic zinc dissolves to form zinc ions, releasing energy; it also loses electrons. If the zinc is connected to the copper via an electric circuit, these electrons can flow around the circuit and reduce copper ions in the lemon.
You have built a battery (or technically a cell), which works on the same principle as the cells you buy in the shops, although these more often use different metals, a more optimised design, and less fruit; but they will all involve a similar reaction that requires electricity to flow for the reaction to occur.
Why are there copper ions in the lemon?
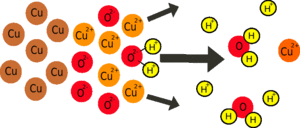
There will be a few naturally, but most of them will be created by the tarnish on the copper dissolving in the acid (see the cleaning copper coins experiment) releasing the copper ions in the copper oxide tarnish).
Why doesn't the reaction happen without the circuit?
If a zinc atom is to dissolve, it must form Zn2+ ions; this involves losing 2 negatively-charged electrons. If these electrons can't go anywhere, the zinc object will become so negatively charged it will attract as many positively charged zinc ions back onto itself as are dissolving, so the reaction will stop.
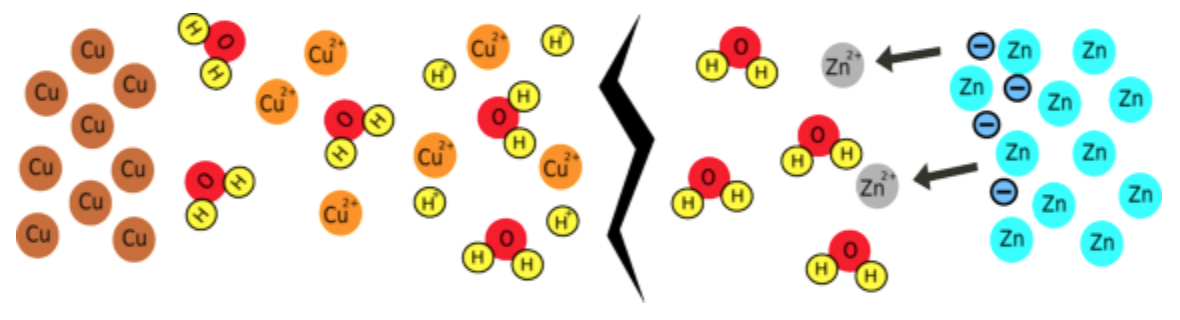
What happens when you complete the circuit?
Electrons can flow around the circuit to the copper electrode; here, copper ions are attracted to the negative electrons and, when they meet, they are reduced, forming copper metal. Because zinc is more reactive than copper, this whole process releases about 1 joule of energy for every coulomb of charge that is moved, which is the same as saying the battery produces about 1 volt. This voltage is related to the difference in reactivity between the two metals you are using, so if you change the metals you will change the voltage.
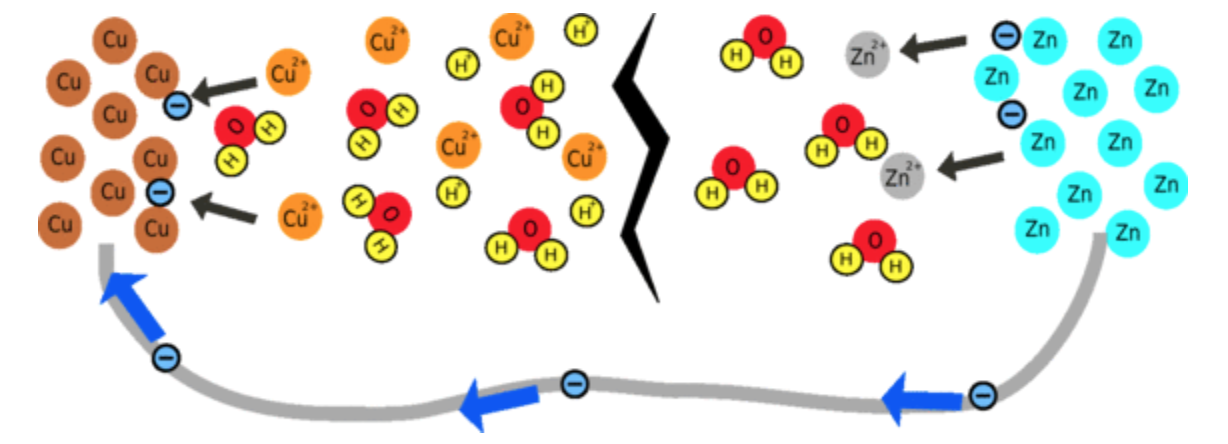
Why does the battery produce such a small current?
To produce a large current, both chemical reactions have to be able to take place quickly, so the larger the surface area and the more reactants there are in the solution, the faster the reaction will occur. There is a very low concentration of Cu2+ ions so the reaction is likely to be quite slow, limiting the current that can be produced.
The other limiting factor is that it is difficult for ions to move around in the fruit. This is because, after a while, the region around the piece of zinc becomes positively-charged and the region around the copper becomes negative. This means that there is less difference in voltage between the zinc and copper nails, so instead of giving out 1V, the cell may only produce 0.5V. But, if you wait for a while, ions will flow through the fruit to cancel out this effect.
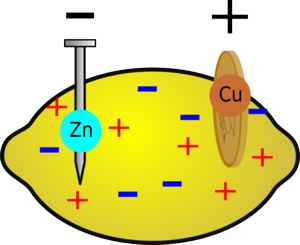
After a while the region around the zinc will become positive due to all the extra zinc ions and the region around the copper will become negative, reducing the voltage of the battery.
But if you wait for a while, without drawing a current, the ions in the fruit will redistribute themselves and the voltage will build up again.
This is why commercial batteries, when they are almost flat, can still produce a good voltage but this drops as soon as you draw a current.
Rechargeable battery
Here we'll create and test a simple rechargeable battery using cardboard, aluminium and copper wire to investigate the principles of energy storage and conversion.
What you'll need:
From your home or school:
- Aluminum foil
- Cardboard (not too thick)
- Saltwater solution (prepared by dissolving salt in water)
From your science set:
- Copper wire
- LED light
- Brad board
- Alligator clips
Optional
- Multimeter (optional, for measuring voltage)
Instructions
Cut small pieces of aluminium foil and copper wire (4-5cm long). Create individual cells by layering aluminium foil then cardboard, and finally copper wire. Use tape to hold the cells together. Connect multiple cells in series by attaching the copper wire of one cell to the aluminium foil of the next cell (you can use alligator clips) Ensure separation between the aluminium foil and copper wire in each cell (that is, they must not touch, the cardboard must separate them)
Next prepare the electrolyte.
An electrolyte is a substance that can conduct electricity when it is dissolved in water or melted.
The electrolyte allows electrical charges to move through it and acts as a pathway for electricity.
When an electrolyte is present, it allows a flow of charged particles called ions. These ions can carry the electric current from one place to another.
Common examples of electrolytes include salt, acids, and bases.
Electrolytes are important in many everyday things like batteries, circuits, and even in our bodies to help our cells and nerves work properly.
Our electrolyte will be salt water. Prepare a saltwater solution by dissolving 2 teaspoons of salt in half a glass of warm water. Make the saltwater solution into a container that will accommodate the battery cells.
Activating our battery:
Now submerge the individual cells of the battery into the saltwater solution, making sure the aluminium foil and copper wire of each cell are immersed but not touching. Allow the battery to sit for a few minutes to allow the chemical reactions to occur. Once the cardboard is soaked you can remove the cells (so long as there is good contact between the aluminium, cardboard and coper). Testing the Rechargeable Battery:
Connect the positive terminal (copper wire) of the battery to the positive terminal of a LED light from your set. Remember the positive terminal of the LED has a longer leg. Connect the negative terminal (aluminium foil) of the battery to the negative terminal of the LED. Observe if the LED light turns on indicating the flow of electrical current. Leave the led connected until it stops working. The battery has now discharged. Note LEDs don't use much power so it may take a long time for it to discharge, you can connect more LEDs in parallel to make it discard faster. You could also connect a small device, like a small electric motor. This will use much more electricity.
Recharging the Battery:
Disconnect the battery from the LED. Connect the positive and negative terminals of the battery to a power source (such as a normal battery) Allow the battery to recharge for a specified period (e.g., a few hours or overnight).
Repeated Testing and Analysis:
Repeat the testing process by reconnecting the recharged battery to the LED. Compare the performance of the battery before and after recharging. Please make observations. If you can measure the voltage is it the same after recharging? Does the LED stay on for as long
Discussion and Reflection:
This is one way to store energy. It's common because it's small and portable, but what are other ways? If you need to store energy from a wind farm - are batteries the best appraoch? How else could you do it?